EVENTS CONVENT HIGH
SCHOOL
16/02/2021 Class-9 SLOT-2
Science
Chapter-4
Structure
of Atoms
_______________________________________
Question 1. What are canal rays?
Answer: Canal rays are positively charged radiations which led
to the discovery of positively charged sub-atomic particle called proton.
Question 2. If an atom contains
one electron and one proton, will it carry any charge or not?
Answer: The atom will be electrically neutral as one – ve charge
balances one + ve charge.
Question 3. On the basis of
Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer: According to Thomson’s model of an atom
(i) An atom consists of a positively charged sphere and the
electrons are embedded in it,
(ii) The negative and positive charges are equal in magnitude.
So the atom is electrically neutral.
Question 4. On the basis of
Rutherford’s model of an atom, which sub-atomic particle is present in the
nucleus of an atom?
Answer: As per Rutherford’s model of an atom, the protons which
are positively charged are present in the nucleus of an atom.
Question 5. Draw a sketch of
Bohr’s model of an atom with three shells.
Answer:
Answer: On using any metal foil, the observations of the
a-particle scattering experiment would remain the same as all atoms would have
same structure.
Question 7. Name the three
sub-atomic particles of an atom.
Answer: The sub-atomic particles of an atom are
Question 8. Helium atom has an
atomic mass of 4 u and two protons in its nucleus. How many neutrons does it
have?
Answer:
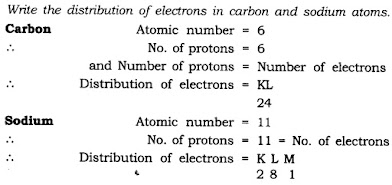
Question 9. Write the distribution of electrons in carbon and sodium atoms.
Answer:
Question 10. If K and L shells of
an atom are full, then what would be the total number of electrons in the atom?
Answer: K shell can hold 2 electrons and L shell can hold 8
electrons.When both the shells are full, there will be (8 + 2) 10 electrons in
the atom.




No comments:
Post a Comment
Thank your for your valuable responce.
Mrfarooqui